- Home
- Clinical Trail & Contract Research
Clinical Trail & Contract Research
Clinical Trails & Contract Research
The contract of Clinical Trial Agreement (CTA) is a legally valid document that manages the relationship between the body that is providing the study drug or device, the financial support and /or proprietary information and the institution that is providing data and/or results, and publication.
A clinical trials agreement should describe and acknowledge responsibilities, terms of collaboration, requirements for payment and reimbursement, publication and intellectual property terms, indemnification and or insurance, subject injury coverage, guidelines for dispute resolution, grounds for termination of contract, and possibility of amending contract terms in the future.
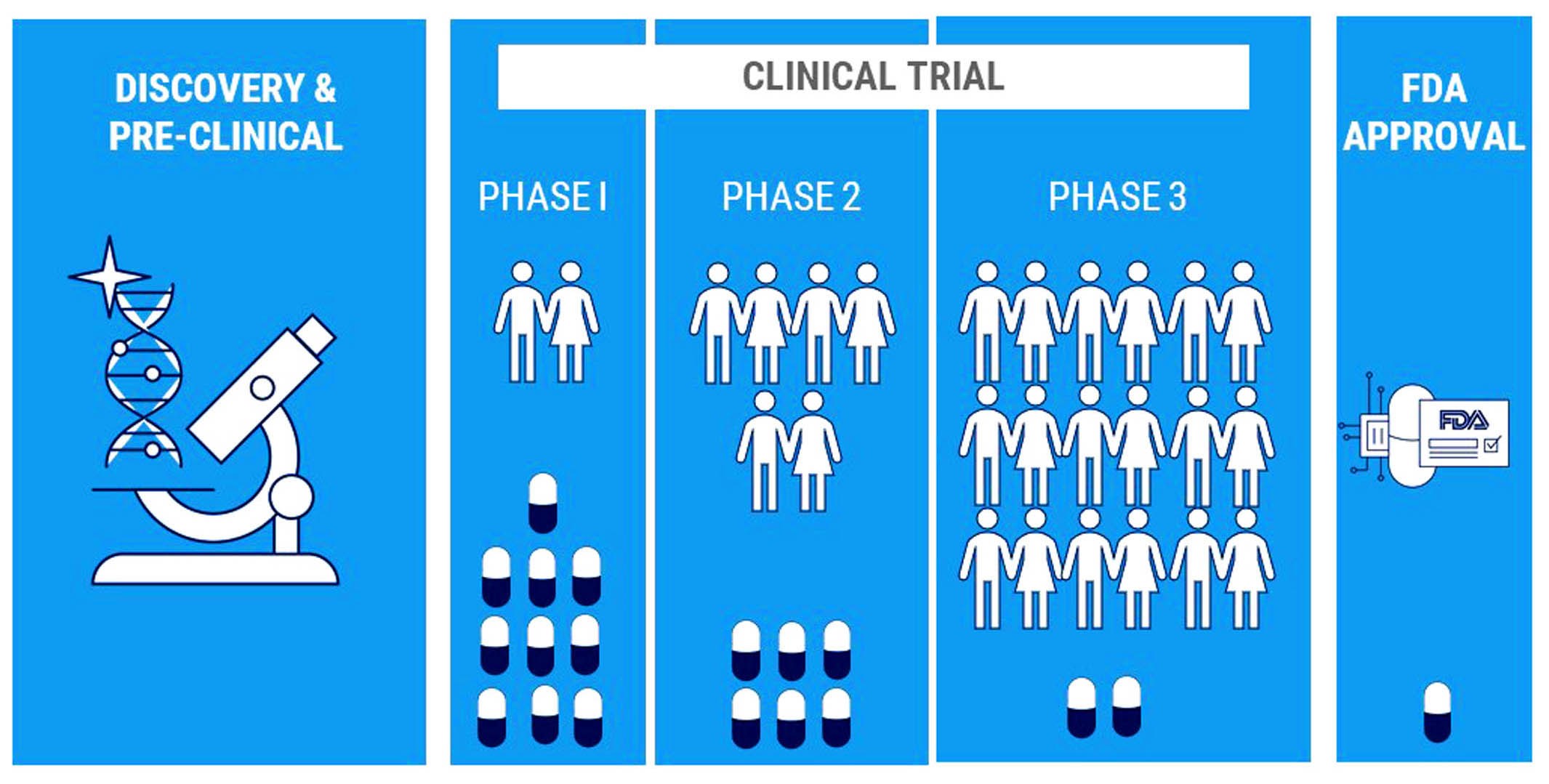
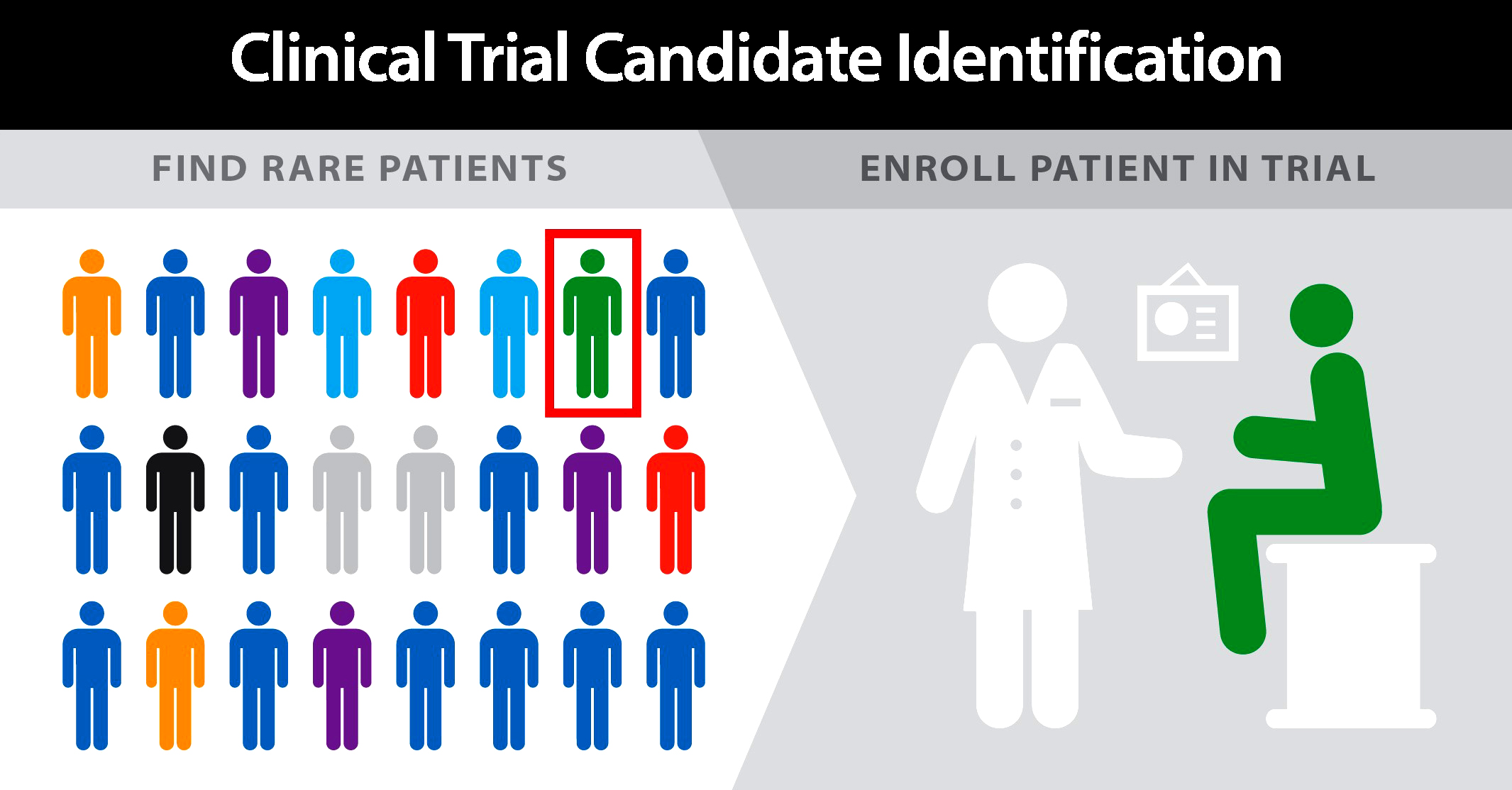
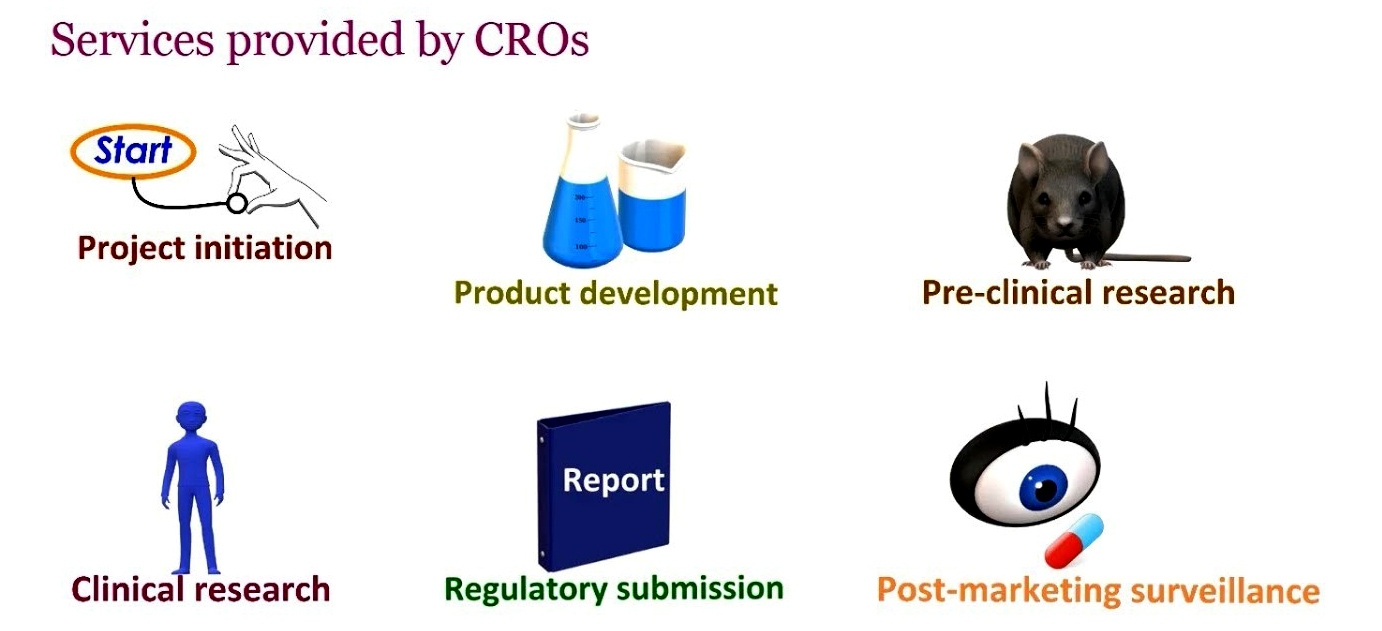
ALS Genomics provides Life Science Contract Research to support Pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on contract basis.
We provide services such as biopharmaceutical development, biological assay development, commercialization, clinical development, clinical trail management, pharmacovigilance, outcome research, and real-world evidence.